Status/Listing
The desert tortoise, a flagship species in the California desert, has been listed as threatened under the California Endangered Species Act since 1989 and the Mojave population has been listed under the Federal Endangered Species Act since 1990 (Figure 1).1 The Sonoran population of tortoises is currently under review for listing by the FWS.2 NatureServe Explorer gives the tortoise a global status of G4 (apparently secure) and a state status of S2 (imperiled) for California.3 The International Union for Conservation of Nature lists the desert tortoise as Vulnerable.4 Despite the listing and attention the species receives for recovery and conservation efforts, populations continue to experience dramatic decline.5
1 U.S. Fish & Wildlife Service, Desert tortoise (Gopherus agassizii), 2010, U.S. Fish & Wildlife Service Species Profile, http://ecos.fws.gov/speciesProfile/profile/speciesProfile.action?spcode=C04L.
2 U.S. Fish & Wildlife Service, Desert tortoise (Gopherus agassizii), 2010, U.S. Fish & Wildlife Service Species Profile, http://ecos.fws.gov/speciesProfile/profile/speciesProfile.action?spcode=C04L.
3 D.J. Morafka and G. Hammerson, “Gopherus agassizzi,” NatureServe Explorer: An online encyclopedia of life [web application]m, 2009, http://www.natureserve.org/explorer.
4 International Union for Conservation of Nature, “Gopherus agassizii,” IUCN Red List of Threatened Species, 2009, http://www.iucnredlist.org/apps/redlist/details/9400/0
5 California Department of Fish and Game, California Wildlife: Conservation Challenges, prepared by the UC Davis Wildlife Health Center for the California Department of Fish and Game, 2005.
Desert Tortoise (Gopherus agassizii)
Habitat Loss
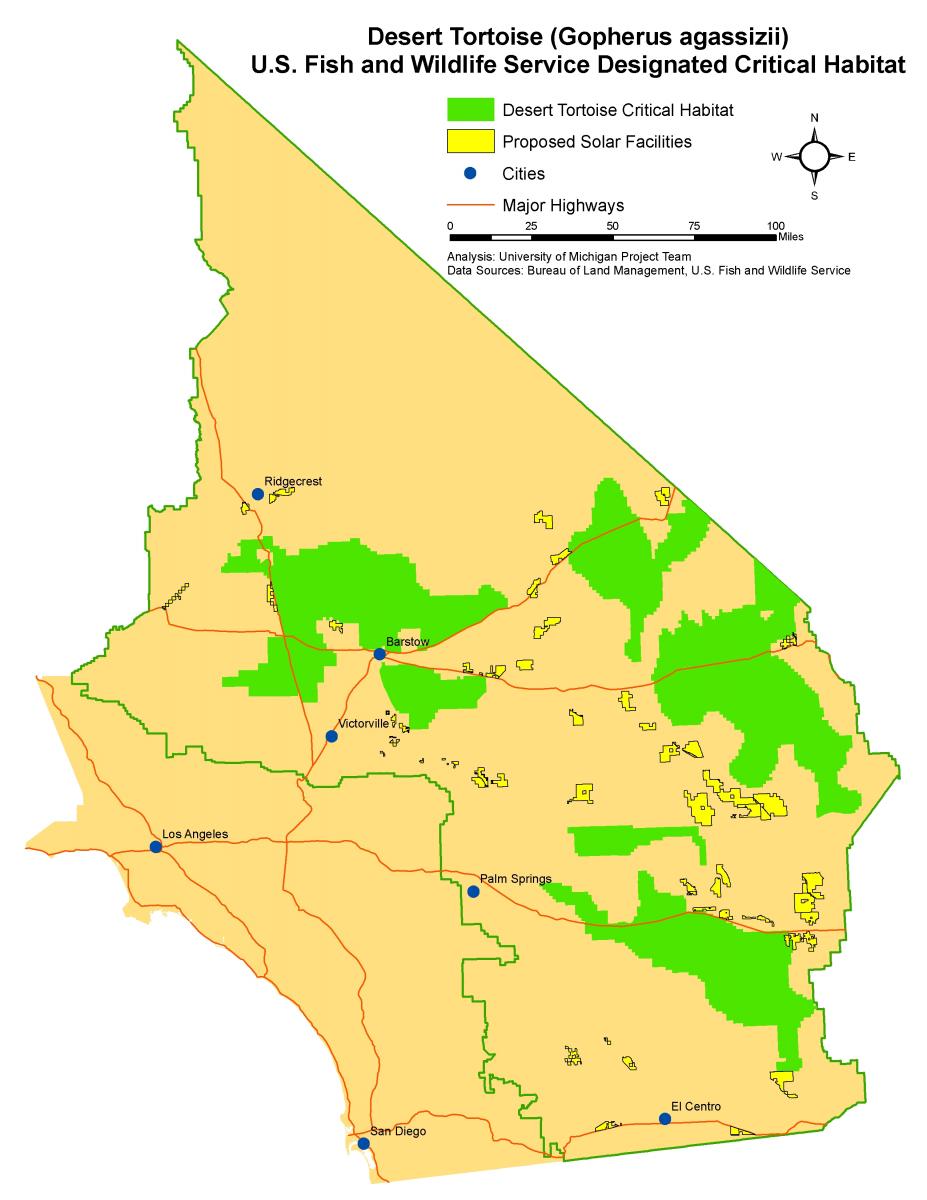
Many solar facilities have proposed siting in or near desert tortoise habitat (Map 1). Development of the these facilities will result in a direct loss of habitat for the desert tortoise because proposed solar facilities plan to use fencing specifically designed to exclude desert tortoises from a project site. Tortoises will be excluded from project sites in order to prevent them from being accidentally killed by machinery during construction, killed by vehicles during operation, or otherwise trapped within the facility. While fencing the entire disturbance area of a facility may prevent the direct mortality of individual tortoises, the total area of habitat that is lost could have negative impacts on tortoise populations, depending on habitat quality within proposed sites.
Surveys of one proposed (2,012 acre) solar facility found zero desert tortoise within the project disturbance area, and five tortoises about 600 feet outside of the proposed disturbance area.1 The low numbers of tortoises found at this site may be due in part to past agricultural activities that left the site heavily disturbed and low in native vegetation. However, surveys of another proposed (1,760 acre) solar facility found 40 desert tortoise within the project disturbance area, and 10 tortoises within the one-mile buffer zone.2 Surveys of the project site and buffer zone also found over 200 tortoise burrows, where 36 active burrows were within the disturbance area and 12 were in the one-mile buffer zone.3 Though the project is not located in designated desert tortoise critical habitat or in a designated desert tortoise Desert Wildlife Management Area (DWMA), the estimated density of adult tortoises within the disturbance area was greater than that of a nearby DWMA: 9.8 desert tortoise per km2 within the proposed solar site, compared with 5.3 to 7.6 desert tortoise per km2 within the nearby DWMA.4 Projects that are built in areas of high desert tortoise density will permanently eliminate large swaths of high quality habitat.
Habitat Fragmentation and Connectivity
Increased habitat fragmentation caused by new facilities, roads, ground disturbance under transmission lines, and other linear corridors (e.g., pipelines), will create barriers to movement that could negatively affect population dynamics. Roads and other linear corridors subdivide contiguous habitat, creating smaller and more isolated tortoise populations. These smaller, isolated populations are more susceptible to decline or local extinction due to drought or other stochastic events, as well as the negative effects of inbreeding. Recovery by small, isolated populations may rely heavily on immigration of new individuals from adjacent habitat, but these inter-population movements are often prevented by the very things that fragmented the habitat in the first place (i.e. roads, development).5
The development of barriers between desert tortoise critical habitats is especially problematic. In a 2009 study, Bare et al. found that solar development in the West Mojave could inhibit movement between certain desert tortoise critical habitats.6 Conserving habitats that allow movement of individuals between critical habitat units is essential to the long-term viability of the Mojave population of the desert tortoise.7,8 Solar development, in addition to eliminating several thousands of acres of occupied and potential desert tortoise habitat per project, may also eliminate or fragment habitats that serve as crucial habitat corridors between conservation areas, which may compromise recovery of the species.
Linear Corridors
New roads constructed to service solar facilities and/or an increase in traffic on existing roads will increase mortality of desert tortoises. Desert tortoises are vulnerable to being run over by automobiles, and the proliferation of new roads through tortoise habitat will likely increase the risk of roadkill mortality.9 Increased OHV access to previously undisturbed natural areas via these new roads could also increase the chances of tortoises being run over by OHVs. In addition to increased OHV access, an increase in human access to tortoise habitat has several other negative implications.
Increased human access to and presence in the California desert due to new solar facilities could benefit the common raven (Corvus corax), considered to be a “subsidized predator” of juvenile desert tortoise.10 Ravens are able to travel long distances to take advantage of human-provided food and water sources at a solar facility, such as trash generated on-site, roadkill created by increased vehicle traffic, and standing water created by dust suppression techniques.11 Raven populations, elevated by human-provided food supplies, venture far beyond developments and into natural areas where they prey on juvenile desert tortoises.12 Solar facility infrastructure, such as fencing, buildings, or transmission lines, could create elevated perches that could be used by ravens to hunt tortoises more effectively. Predation by ravens is a serious threat to desert tortoise populations. In a 2003 study by Kristan and Boarman, the authors state that “anthropogenic resources for ravens could indirectly lead to the suppression, decline, or even extinction of desert tortoise populations.”13 More recently, coyote (Canis latrans) predation on desert tortoise has become a problem especially in light of the prolonged drought being experienced in the region and a decline in prey species, such as jack rabbits and ground squirrels.14 A proliferation of solar development in desert tortoise habitat could supplement existing human-provided resources for ravens and coyotes, and lead to further decline of tortoise populations.
The proliferation of new roads in or near tortoise habitat has the potential to negatively affect tortoise populations via increased trash or litter, collection, disease, and vandalism. Increased human access to desert areas in the proximity of tortoise habitat could lead to an increase in the amount of trash or litter in tortoise habitat. Tortoises have been known to eat balloons, plastic, and other pieces trash, which can become lodged in the digestive tract, eventually causing death.15 Other human activities that endanger tortoise populations are collection of tortoises (i.e. for pets), release of previously- collected tortoises from captivity that can spread diseases to wild tortoises, and vandalism.16,17 Disease in wild desert tortoise populations is responsible for increased stress and mortality in tortoises, likely contributing to population declines.18,19
Invasive Plants
Roads and other transportation corridors necessary for solar development could facilitate the colonization of natural areas by invasive plants.20 The spread of invasive plants is problematic for the desert tortoise because of its effect on the frequency of fire. Invasive grasses increase the frequency of fire by increasing the amount of vegetative fuel and by reducing the space between plants, allowing fire to spread to a larger area.21 Wildfires have both direct and indirect effects on tortoises. The slow- moving tortoises might not be able to escape a fast-moving wildfire, and could therefore suffer direct mortality in a fire.22 If a tortoise does survive a fire, the loss of vegetation from the fire could leave large areas devoid of food for the tortoise and lead to starvation. In addition, loss of vegetative cover leads to loss of protection from predators (i.e. places to hide) and temperature extremes (i.e. loss of shade).23
Implications for the Ecosystem
To escape from harsh environmental conditions, tortoises will utilize a wide variety and number of burrows that they have either excavated on their own or modified from another animal.24 Excavation and construction of burrows by tortoises can provide habitat for several other species, including, but not limited to: antelope ground squirrel (Ammospermophilus lecurus), blacktailed jackrabbit (Lepus californicus), kangaroo rat (Dipodomys spp.), kit fox (Vulpes macrotis), burrowing owl (Athene cunicularia), Gambel’s quail (Callipepla gambelii), roadrunner (Geococcyx californianus), desert spiny lizard (Sceloporus magister), western rattlesnake (Crotalus viridis), ground beetle (Tenbrionidae), and tarantula (Aphonopelma spp.).25 Because the tortoise creates microhabitats for numerous other species, it could be considered a keystone species of the California desert.26 Extirpation or continued decline of the desert tortoise in the California desert from the impacts discussed above may have implications for species that currently benefit from tortoise burrows.
Ecological Implications for Facility Location and Design
The magnitude of impact that the development of a single solar facility or multiple facilities will have on the desert tortoise is dependent on the facility design variables and location as discussed in Table 1. These variables will influence the magnitude of the cumulative impact of solar development on the desert tortoise populations in the California desert.
| Facility Design Variables | Implications for the Desert Tortoise |
|---|---|
| Location of Facility | Determines the quality of desert tortoise habitat that is eliminated (some areas are better quality tortoise habitat than others). Determines the magnitude of habitat fragmentation (some areas are more heavily hused by tortoises for movement and migration). |
| Size of Facility | Larger facilities have a greater probability of eliminating desert tortoise habitat. |
| Proximity to other development | The closer a facility is to developed areas, the more likely it is that desert tortoise habitat is already degraded and populations are depressed from predation and other human impacts. |
| New and existing roads to access the facility | More roads increase the probability of roadkill mortality. |
| Number of construction and operation personnel | More vehicles increase the probability of roadkill mortality. |
| Speed limits | Lower speed limits reduce the probability of roadkill mortality. |
| Length of new transmission line(s) | Longer transmission lines create a larger disturbance area and increase habitat fragmentation. |
| On-site Raven Management Plan | An effective raven management plan might prevent the establishment of ravens at the facility site and could reduce predation of ravens on desert tortoise. |
| On-site trash and standing water BMPs | Secured trash and minimization of standing water on-site reduces attractiveness of the facility to predators (e.g. ravens, coyotes) and reduces predation on desert tortoise. |
| Invasive plant and fire management plans | Plans that minimize the establishment and spread of invasive plants and contain fires reduce direct and indirect mortality of tortoises from fire. |
1 California Energy Commission, Application for Certification [project and applicant name withheld], 2010, http://www.energy.ca.gov/sitingcases.
2 California Energy Commission, Application for Certification [project and applicant name withheld], 2010, http://www.energy.ca.gov/sitingcases.
3 California Energy Commission, Application for Certification [project and applicant name withheld], 2010, http://www.energy.ca.gov/sitingcases.
4 California Energy Commission, Application for Certification [project and applicant name withheld], 2010, http://www.energy.ca.gov/sitingcases.
5 T.E. Edwards, C.R. Schwalbe, D.E. Swann, and C.S. Goldberg, “Implications of Anthropogenic Landscape Change on Inter- Population Movements of the Desert Tortoise (Gopherus agassizii),” Conservation Genetics 5, no. 4 (2004): 485-499.
6 L. Bare, T. Bernhardt, T. Chu, M. Gomez, C. Noddings, and M. Viljoen, “Cumulative Impacts of Large-scale Renewable Energy Development in the West Mojave,” (Santa Barbara, CA: Donald Bren School of Environmental Science & Management, University of California, Santa Barbara, 2009), http://fiesta.bren.ucsb.edu/~westmojave/proposal.html.
7 T.E. Edwards, C.R. Schwalbe, D.E. Swann, and C.S. Goldberg, “Implications of Anthropogenic Landscape Change on Inter- Population Movements of the Desert Tortoise (Gopherus agassizii),” Conservation Genetics 5, no. 4 (2004): 485-499.
8 U.S. Fish and Wildlife Service Staff Member 3, Personal Communication, April 6, 2010.
9 M.C. Grover and L.A. DeFalco, “Desert Tortoise (Gopherus agassizii): Status-of-Knowledge Outline With References,” U.S. Department of Agriculture Intermountain Research Station, 1995.
10 W.B. Kristan and W.I. Boarman, “Spatial Pattern of Risk of Common Raven Predation on Desert Tortoises,” Ecology 84, no. 9 (2003): 2432-2443.
11 W.I. Boarman, “Managing a subsidized predator population: Reducing common raven predation on desert tortoises,” Environmental Management 32, no. 2 (2003): 205-217.
12 W.I. Boarman, “Managing a subsidized predator population: Reducing common raven predation on desert tortoises,” Environmental Management 32, no. 2 (2003): 205-217.
13 W.B. Kristan and W.I. Boarman, “Spatial Pattern of Risk of Common Raven Predation on Desert Tortoises,” Ecology 84, no. 9 (2003): 2432-2443.
14 U.S. Fish and Wildlife Service Staff Member 3, Personal Communication, April 6, 2010.
15 U.S. Fish & Wildlife Service, Desert Tortoise (Mojave Population) Recovery Plan, 1994, U.S. Fish & Wildlife Service, Portland, OR.
16 U.S. Fish & Wildlife Service, Desert Tortoise (Mojave Population) Recovery Plan, 1994, U.S. Fish & Wildlife Service, Portland, OR.
17 Jody Fraser, Research Biologist, U.S. Fish & Wildlife Service, Personal Communication, January 22, 2010.
18 B.L. Homer, K.H. Berry, M.B. Brown, G. Ellis, and E.R. Jacobson, “Pathology of diseases in wild desert tortoises from California,” Journal of Wildlife Diseases 34, no. 3 (1998): 508-523.
19 M.M. Christopher, K.H. Berry, B.T. Henen, and K.A. Nagy, “Clinical disease and laboratory abnormalities in free-ranging desert tortoises in California (1990-1995),” Journal of Wildlife Diseases 39, no. 1 (2003): 35-56.
20 M.L. Brooks, “Spatial and Temporal Distribution of Nonnative Plants in Upland Areas of the Mojave Desert,” in The Mojave Desert: Ecosystem Processes and Sustainability, ed. R.H. Webb, L.F. Fenstermaker, J.S. Heaton, D.L. Hughson, E.V. McDonald, and D.M. Miller, (Reno: The University of Nevada Press, 2009), 101-124.
21 M.L. Brooks and J.R. Matchett, “Spatial and temporal patterns of wildfires in the Mojave Desert, 1980-2004,” Journal of Arid Environments 67 (2006): 148-164.
22 T.C. Esque, C.R. Schwalbe, L.A. DeFalco, R.B. Duncan, and T.J. Hughes, “Effects of Desert Wildfires on Desert Tortoise (Gopherus agassizii) and Other Small Vertebrates,” The Southwestern Naturalist 48, no. 1 (2003): 103-111.
23 T.C. Esque, C.R. Schwalbe, L.A. DeFalco, R.B. Duncan, and T.J. Hughes, “Effects of Desert Wildfires on Desert Tortoise (Gopherus agassizii) and Other Small Vertebrates,” The Southwestern Naturalist 48, no. 1 (2003): 103-111.
24 M.C. Grover and L.A. DeFalco, “Desert Tortoise (Gopherus agassizii): Status-of-Knowledge Outline With References,” U.S. Department of Agriculture Intermountain Research Station, 1995.
25 M.C. Grover and L.A. DeFalco, “Desert Tortoise (Gopherus agassizii): Status-of-Knowledge Outline With References,” U.S. Department of Agriculture Intermountain Research Station, 1995.
26 Mark Massar, Wildlife Biologist, Bureau of Land Mangement, Personal Communication, November 5, 2009.