Research
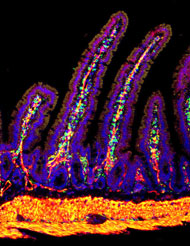 Cell: cell communication during the development of the small intestine
Cell: cell communication during the development of the small intestine
The goal of this project is to learn more about the constant signaling crosstalk between intestinal epithelium and mesenchyme that acts to pattern the intestine. In earlier studies, we demonstrated that Indian and Sonic Hedgehog, secreted from the intestinal epithelium, are required for patterning both the mesenchyme and the epithelium itself. Mesenchymal patterning is due to a direct paracrine hedghog signal. Events downstream of hedgehog signals include the localization of Wnt-secreting myofibroblasts beneath the pre-crypt regions of the intestine and the generation of smooth muscle structures in the cores of the villi. Epithelial patterning downstream of hedgehog signals is indirect and is controlled by secondary signals secreted from the mesenchyme, including Wnt, Bmp, and others. The location of the epithelial proliferative compartment is controlled in this way. In addition, we recently found that the generation of intestinal villi is a hedgehog-dependent morphogenic event. Using microarray, we are identifying the specific genes in mesenchymal cells that are targets for the hedgehog signal, concentrating on those genes that encode products that could generate secondary signals which impact epithelial function.
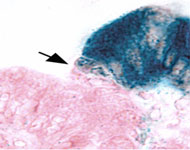 Patterning of the epithelial pyloric border
Patterning of the epithelial pyloric border
We are trying to understand how an intestinal cell "decides" to be intestine rather than stomach. We have identified the precise time during mouse development at which a distinct border forms in the previously un-patterned epithelium, separating stomach from intestine. The process that regulates this identity decision may be altered in cases of intestinal metaplasia, lesions of the stomach in which the stomach epithelium takes on the appearance of intestine. Intestinal metaplasia is associated with the development of gastric cancer, making this identity program a critical target for further research.
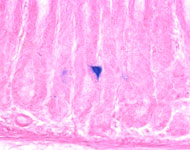 Characterization of a novel stomach stem cell population
Characterization of a novel stomach stem cell population
We have found the first marker by which a rare type of stomach stem cell can be prospectively recognized and isolated from the adult stomach. We have shown that these cells have multilineage potential and that they can give rise to all of the cells in a gastric gland. Interestingly, this cell population is greatly increased during inflammation or by the pro-inflammatory cytokine, interferon gamma. Because the gastric stem cell compartment is the likely target of mutations that promote tumors, and because tumorigenesis in the stomach is highly associated with chronic inflammation, we expect that the further characterization of this cell population will have major implications for gastric cancer.